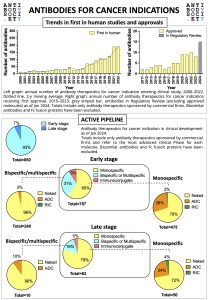
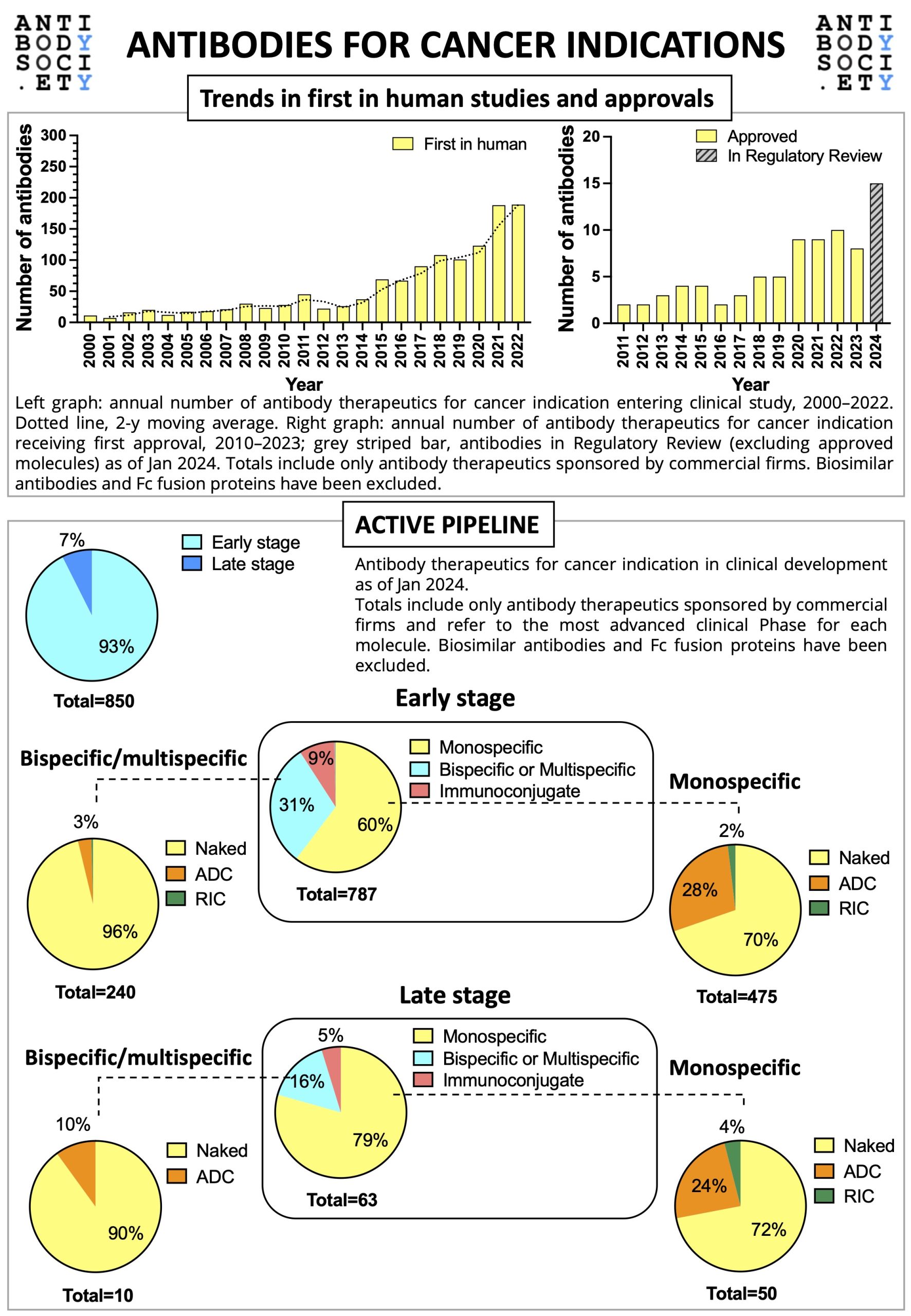
For World Cancer Day 2024, The Antibody Society has prepared a snapshot of the clinical development of therapeutic antibodies for cancer indication.
The infographic gives an overview on the trends in first in human studies and approvals, as well as on the active early and late stage pipelines (as of January 2024).



 Immunomedics announced the issuance of a novel patent (U.S. Patent 9,375,489) related to the company’s lead cancer therapeutic, sacituzumab govitecan, also known as IMMU-132. This antibody-drug conjugate (ADC) comprises a humanized antibody to the cancer target Trop-2 and is conjugated with SN-38, an active metabolite of the anti-cancer drug irinotecan. The patent entitled “Antibody-SN-38 Immunoconjugates with a CL2A Linker.” is the 28th issued U.S. patent covering the uses and composition of sacituzumab govitecan.
Immunomedics announced the issuance of a novel patent (U.S. Patent 9,375,489) related to the company’s lead cancer therapeutic, sacituzumab govitecan, also known as IMMU-132. This antibody-drug conjugate (ADC) comprises a humanized antibody to the cancer target Trop-2 and is conjugated with SN-38, an active metabolite of the anti-cancer drug irinotecan. The patent entitled “Antibody-SN-38 Immunoconjugates with a CL2A Linker.” is the 28th issued U.S. patent covering the uses and composition of sacituzumab govitecan.
