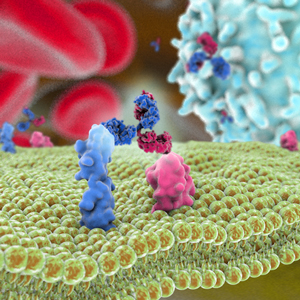
 On January 28, 2022, Genentech announced that the U.S. Food and Drug Administration has approved Vabysmo ™ (faricimab-svoa) for the treatment of wet, or neovascular, age-related macular degeneration (AMD) and diabetic macular edema (DME). Faricimab (RO6867461, RG7716) is an anti-vascular endothelial growth factor-A (VEGF-A) and anti-angiopoietin-2 (Ang-2) bispecific antibody derived from Roche’s CrossMab technology.
On January 28, 2022, Genentech announced that the U.S. Food and Drug Administration has approved Vabysmo ™ (faricimab-svoa) for the treatment of wet, or neovascular, age-related macular degeneration (AMD) and diabetic macular edema (DME). Faricimab (RO6867461, RG7716) is an anti-vascular endothelial growth factor-A (VEGF-A) and anti-angiopoietin-2 (Ang-2) bispecific antibody derived from Roche’s CrossMab technology.
The approval was based in part on results from four Phase 3 studies in wet AMD and DME. The randomized, double-masked, and active comparator-controlled TENAYA (NCT03823287) and LUCERNE (NCT03823300) studies evaluated the effects of faricimab (6.0 mg administered at fixed intervals of every two, three, or four months) and aflibercept (Eylea®) (2.0 mg administered at fixed two-month intervals) in wet AMD patients. The primary endpoint of the studies, average change in best-corrected visual acuity (BCVA) from baseline through week 48, was met in both studies. The average vision gains from baseline in the faricimab arms were +5.8 and +6.6 letters, compared to +5.1 and +6.6 letters in the aflibercept arms, in the TENAYA and LUCERNE studies, respectively, demonstrating the non-inferiority of faricimab compared to aflibercept. The study also showed that faricimab’s treatment interval could be longer than that of aflibercept – nearly 80% of patients treated with faricimab were able to go three months or longer between treatments during the first year.
The 3-arm, randomized, double-masked, active comparator-controlled YOSEMITE (NCT03622580) and RHINE studies (NCT03622593) compared the effects of faricimab (6.0 mg administered at personalized treatment intervals (PTI) of up to four months or 6.0 mg administered at fixed two-month intervals) to those of aflibercept (2.0 mg administered at fixed two-month intervals) in DME patients. The primary endpoint, average change in BCVA score from baseline at one year, was met, with faricimab again showing non-inferiority in visual acuity gains compared to aflibercept. In the YOSEMITE study, the average vision gains from baseline were +11.6, +10.7, and +10.9 letters eye chart letters in the faricimab PTI, faricimab two-month, and aflibercept arms, respectively. The average vision gains from baseline were +10.8, +11.8, and +10.3 letters in the faricimab PTI, faricimab two-month, and aflibercept arms, respectively, in the RHINE study.
Further details for the TENAYA and LUCERNE and YOSEMITE and RHINE studies were published in The Lancet.
The European Medicines Agency has accepted the submission of a Marketing Authorisation Application for faricimab.
Vabysmo ™ is the 2nd antibody-based therapeutic granted a first approval for marketing in the EU or US in 2022. Explore our searchable table of antibody therapeutics approved in the EU or US for details.