Registration is open!
Trends in the commercial development of antibody therapeutics: Focus on the early-stage pipeline
Tuesday October 24th, 11am ET/4pm BST/5pm CET
Speaker: Silvia Crescioli, PhD (The Antibody Society)
Need insights into the early-stage antibody therapeutics pipeline? We’ve got you covered! Join us Tuesday Oct 24th for an in-depth analysis of the early-stage pipeline stratified by cancer and non-cancer indications, revealing trends in the molecular formats, targets, and mechanism of action.
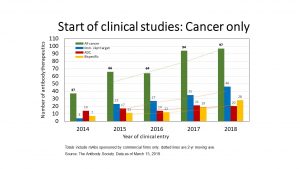
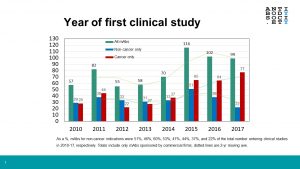
Abstract: Since 2014, the number of antibody therapeutics entering clinical development annually has increased steadily, from 71 in 2014 to 286 in 2022. This has resulted in a clinical pipeline currently composed of ~1250 molecules, of which ~1100 and ~150 molecules are in early- and late-stage development, respectively. Despite the great interest in trends in early-stage clinical development, due to the difference in scale and difficulty in tracking molecules newly entered in clinical studies, analyses of trends in the global commercial development of antibody therapeutics are often limited to the late-stage clinical pipeline only. Luckily, The Antibody Society meticulously collects data for antibody therapeutics at all stages of clinical development. This webinar will provide an exhaustive analysis of the early-stage pipeline stratified by cancer and non-cancer indications, revealing trends in the molecular formats, targets, and mechanism of action.