Thank you for joining us at The Antibody Society’s 2nd annual Antibody Engineering & Therapeutics Europe meeting (AE&TE) June 11-13 2019. The meeting was a great opportunity for Society members to connect with industry and academic scientists and executives from Europe and around the world focused on antibody and protein therapeutic discovery and development.
As always, The Antibody Society’s members designed the scientific program and acted as session Chairs. The meeting featured sessions on Turning Antibody Leads into Drugs, Recent Advances in Immuno-Oncology Approaches, Bioinformatics and Repertoires in Antibody Discovery and Development, Antibody Therapeutics for Autoimmune and Neurodegenerative Diseases, Clinical Developments in Antibody Therapeutics, and New Antibody Formats and Effector Functions.
AE&TE Student/Postdoc Poster Competition
- Antibody Society Student/Postdoc Poster Competition Winner Roberta Lotti
- Antibody Society Student/Postdoc Poster Competition Winner Amiram Sananes
- Amiram Sananes with poster and award
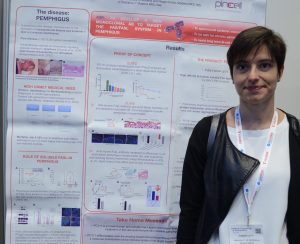
- Roberta Lotti with poster
- Closeup of award from The Antibody Society
The Society sponsored a poster competition for students and postdocs, with winners receiving complimentary registration, support for travel expenses, and an opportunity to present at the conference. Congratulations to the winners, Roberta Lotti and Amiram Sananes!
“I would really thank The Antibody Society for giving me this opportunity to grow and enlarge my research horizon.This award gave me the opportunity to get in touch with a new research community and, as I hope, to enlarge my contacts and my collaborations. ” – Roberta Lotti
“I believe it’s important for students to attend these conferences and come out from their shell once in a while, and in doing so get new ideas while enjoying sunny Amsterdam!” – Amiram Sananes
The Antibody Society’s Networking Session
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
- Networking session at The Society’s booth
The Society hosted a networking session at our booth during the meeting.
Society Sponsors
- Twist Bioscientific booth
Society sponsors ImmunoPrecise, Aldevron, Twist Bioscience, and Geneious Biologics were present at the meeting.
We look forward to seeing you at AE&TE in 2020!
- Kerry Chester, Session Chair and Antibody Society Board Member
- Michael Keenan at the podium
All Society members receive discounts on registration for AE&TE, Antibody Engineering & Therapeutics (US), as well as registrations for many antibody-related meetings.